Thomas Reed (1), Jim Fournier (1) and Greg Nelson (2)
(1) Biochar Energy Corporation, Golden, CO, USA (2) Peak Analytical, Inc., Golden, CO, USA
The names “charcoal” and “biochar” cover a multitude of “charcoal” compositions made at temperatures from 300-1000ºC for various purposes such as cooking, heating, power, and now agricultural soil enhancement and carbon sequestration. Many groups are investigating soil enhancement with uncharacterized charcoal and others are manufacturing charcoal, again mostly uncharacterized.
The Biochar Energy Corporation is developing charcoal manufacture by several fundamentally different methods. We believe it is necessary to characterize the different grades of “charcoal” for research and eventual application purposes. The major classification of “charcoals” is into:
- Charcoals which retain some of the original biomass bonding (torrefied wood);
- Those charcoals which have been prepared at a high enough temperature to destroy most of the original bonds
- Those charcoals which have been allowed to re-absorb the volatiles (vinegar) which are initially generated at higher temperature.
Raman spectroscopy is a spectroscopic technique used in condensed matter chemistry to study vibrational, rotational, and other low-frequency modes in a compound. It relies on inelastic or Raman scattering of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range. The laser light interacts with phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the phonon modes in the system. Infrared spectroscopy yields similar, but complementary information. We also took IR spectra of the same samples and will report on them at another time.
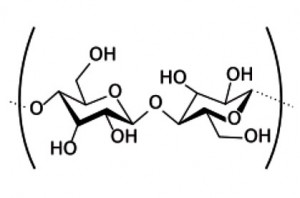
Tetrahedral bonding in cellulose
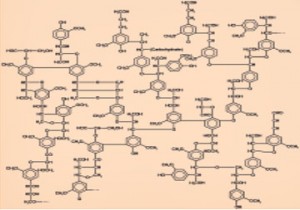
Graphitic bonding in Lignin
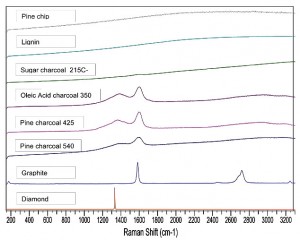
Wood and biomass are typically composed of ~75% cellulose and hemicelluloses which exhibit tetrahedral carbon bonding as shown above. However, the remaining 25% lignin is formed on a benzene ring and so has planar graphitic bonds (above). To characterize the degree of the changes during pyrolysis, we present here the Raman shift spectra (RSS) of five biomass samples pyrolysed from 215ºC (sucrose) to 540ºC (Pine) and showing their transition to increasing graphitic bonding as temperature rises.
The Raman shift spectra (RSS) of both pine wood and lignin show no peak due to fluorescence. The RSS of pure graphite on the other hand shows two very strong peaks at ~ 1600 and 2700 cm-1 (2720 and 1582 cm-1). The peak at 1600 cm-1 is the graphite (G band) first order phonon originating from the E2g mode of C-C stretching of the aromatic structure. The diamond spectrum at the bottom of the graph shows only the tetrahedral bonding peak at 1400 cm-1 characteristic of cellulose and is indicative of incomplete destruction of the original biomass structure.
Sugar (sucrose) was pyrolysed on a hot plate at 215ºC to produce a very low temperature charcoal. The 1600 cm-1 peak of graphite is just barely showing (at higher magnification), suggesting a very small degree of pyrolysis to charcoal even at this low temperature.
Oleic acid was pyrolysed at 350ºC and shows two strong peaks at 1400 and 1600 cm-1. The peak at 1600 cm-1 is identical to the major peak of graphite, as well as a secondary peak at 1400 cm-1 which is characteristic of disordered graphite. Pine wood chips were pyrolysed in a top lit updraft gasifier at 425ºC and 540ºC. Both peaks are well developed, but integration of the peak areas show that the ratio of the graphite peak at 1600cm-1 to the disordered graphite peak at 1400 cm-1 has decreased about 7% at 540ºC from the ratio at 425ºC.
This survey of bonding shows that charcoals made over a range of temperatures show characteristic peaks which correlate with the degree of conversion of tetrahedral carbon bonds in cellulose to graphitic bonds in charcoals. While most of the charcoal resulting from pyrolysis of biomass probably derive from the ~25% lignin content, the spectra of sucrose and oleic acid charcoal show that some of the charcoal can derive from tetrahedrally bonded aliphatic compounds.



 Peak Analytical's Gamma Program is designed to help you prevent downtime and, in the event of a breakdown, mitigate your downtime losses with Fast Turnaround Analysis at affordable rates.
Peak Analytical's Gamma Program is designed to help you prevent downtime and, in the event of a breakdown, mitigate your downtime losses with Fast Turnaround Analysis at affordable rates.